Gene Therapy refers to the introduction of foreign normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes for therapeutic purposes. It has shown great therapeutic potential in various diseases such as cancer, hereditary diseases such as thalassemia, sickle anemia, hemophilia and congenital blackness. The unique potential of gene therapy to cure a genetic disease for a lifetime can make everything impossible.
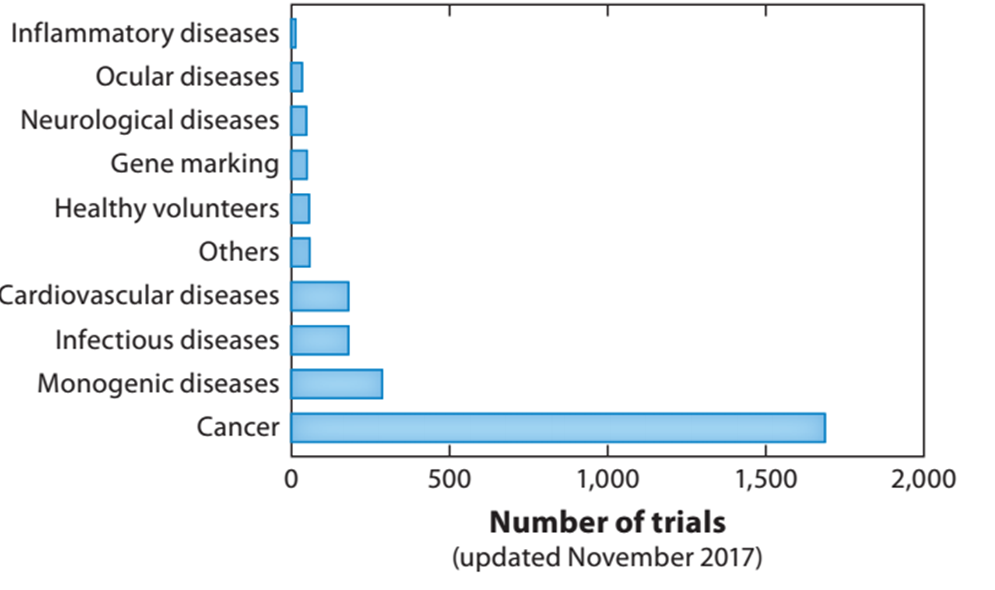
Cancer and rare diseases are currently the most focused areas of gene therapy. According to Pharma projects' report in 2018 [1], the current gene therapy category mainly involves: cancer, rare genetic diseases, cardiovascular diseases, infectious diseases, etc. Cancer and rare diseases are the two most recent clinical studies in gene therapy. The figure below shows the number of clinical trial indications for gene therapy as of 2017.
How is gene therapy classified?
Gene therapy in a broad sense refers to the method of transferring certain genetic material into a patient, expressing it in the body, and finally reaching a certain disease. Narrow gene therapy refers to the way in which genes that function normally function to correct or replace the original defective genes for therapeutic purposes.
At present, gene therapy is mainly divided into the following three categories, including classification according to treatment route, mode of operation and target cells.
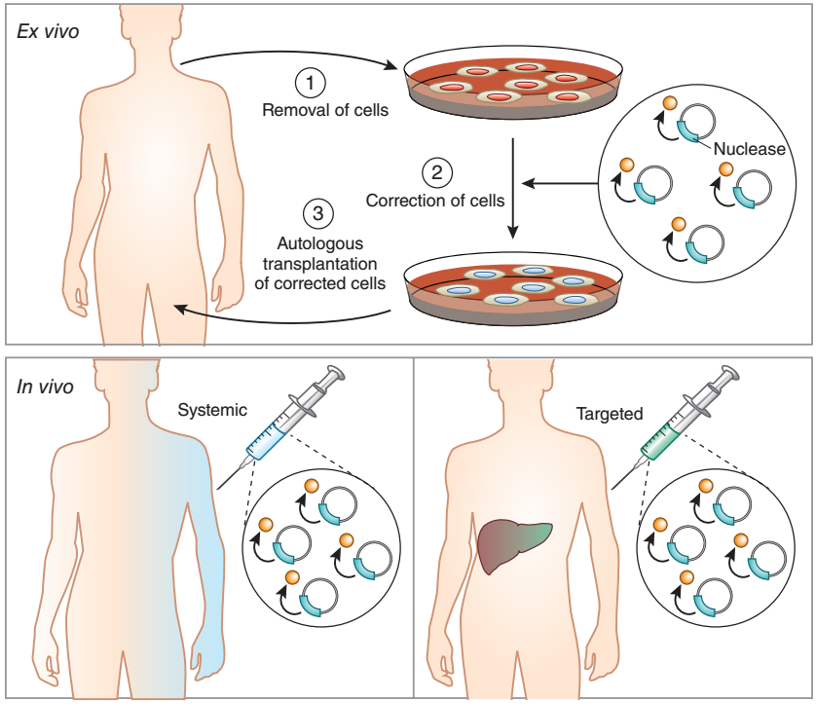
First, according to the route of drug delivery can be divided into two kinds of in vivo and in vitro gene therapy (Figure 2):
1. In vivo ( in situ cell gene therapy): The treatment of a disease by directly delivering a therapeutic gene to a patient's disease site using a non-viral or viral vector (either viral-based and gene-editing-based). This method is convenient to operate and is the best choice for some cell types that cannot be cultured in vitro. However, due to the uncertainty of in vivo editing, many events such as random integration or off-target are unavoidable, and some viral vectors can cause life-threatening immune responses in the body.
2. Ex vivo ( ex vivo ) (the patient's cells are modified in vitro and then transplanted back) : directly take the patient's own diseased cells, and modify the diseased cells by gene introduction in vitro to enable high-efficiency expression of therapeutic proteins. It is then expanded in vitro and finally returned to the patient for the purpose of treating the disease. This method can not use immune cells such as immune rejection in the use of autologous cells, and can also screen highly efficient transduction and off-target cells in vitro to achieve efficient and safe gene therapy. However, due to the cumbersome steps, the shortcomings of cell viability are also limited.
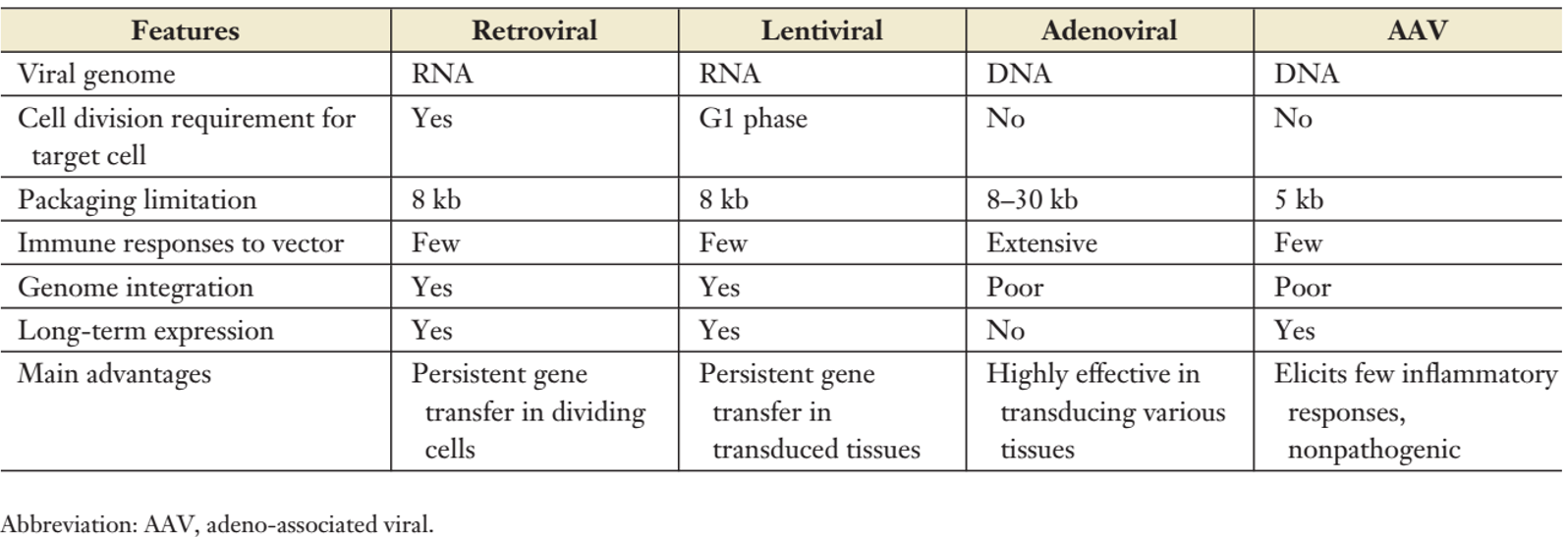
One of the main distinguishing factors in gene therapy in vitro and in vivo is the choice of vector (Figure 3). As described above, the vector can be classified into an integrated vector and a non-integrated vector . So how to choose?
l When introducing genetic material into stem cells, the integration vector is preferred, so that the donor DNA will be integrated into the genome of the stem cell and will be replicated during cell division, so that the genetic material can be transmitted to all daughter cells. .
For in vivo gene therapy, it is usually targeted to cells after mitosis. In these cells, since they do not divide again, long-term expression can be achieved as long as the introduced foreign gene is stably present in the cell; the stable episome is sufficient to drive the periodic expression of the foreign gene.
Most current gene therapy strategies for genetic diseases focus on two vectors:
1) Lentiviral (L V ) vectors for in vitro gene transduction into hematopoietic and other stem cells : such as bluedird Bio; LentiGlobin is a lentiviral vector-based in vitro gene therapy that shows great potential, However, the semi-random vector integration method has the risk of carcinogenesis. At the same time, the expression components in the lentivirus will gradually silence during the long-term homing and self-renewal of hematopoietic stem cells, which will reduce the curative effect and may not achieve the goal of lifelong cure. And this method requires a lot of viruses, which means that the huge cost is high.
2) Adeno-associated virus (A AV ) vectors for in vivo gene transduction to post-mitotic cell types : Luxturna, such as Spark, is an approved hereditary retinopathy for the treatment of RPE65 gene defects in 2017. The normal RPE65 gene is carried by the AAV virus and injected into the retina. The normal RPE65 gene is not integrated into the DNA of human cells, but the normal RPE65 protein is synthesized in the nucleus, thereby triggering the light-conducting pathway and restoring normal visual function. .
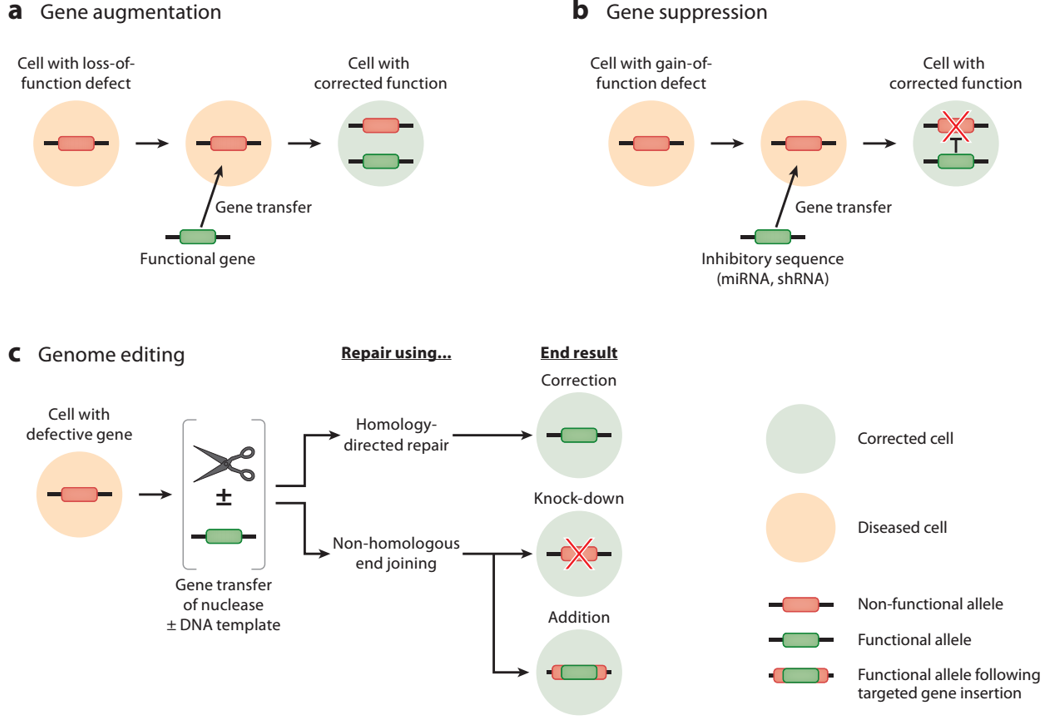
Second, according to the way of genetic operation, it is divided into two categories (Figure 4 ):
1. The gene enhancer (gene augmentation) and gene inactivation (gene suppressi on): to express normal products by introducing a foreign gene, thereby compensating for defective function of the gene or the like, in addition to abnormal gene not required; or specific blocking of certain genes Translation or transcription to achieve inhibition of the expression of certain abnormal genes.
2. Edit gene (Gen ome editing), divided into correct gene (gene correction), knockout (gene knockout) was added and the gene (gene a d dtion): techniques by site-directed integration of the exogenous gene at a specific location normally The point is replaced or inserted to correct the abnormal sequence of the defective gene and to repair it in situ without any other changes in the genome.
According to the different target cells, it can be divided into two types: somatic cells and germ cells:
1. somatic gene therapy (S omatic cell) refers to the transfer of the normal gene into a somatic cell, so that expression of the gene product for therapeutic purposes. This method generally involves introducing a foreign normal gene into a chromosome-specific gene locus in a target cell, replacing the abnormal gene with a healthy gene, thereby exerting a therapeutic effect, and also reducing the possibility of random insertion causing a new gene mutation. There are still great difficulties in the fixed integration of the genome.
2. The germ cell gene therapy (G erm cell) is directly introduced into the germ cells of a normal gene to correct the defective gene. Not only can genetic diseases be treated in the contemporary era, but new genes can be passed on to future generations of patients, and genetic diseases can be cured. However, gene therapy of germ cells involves more problems and more complicated techniques. Therefore, more somatic gene therapy is now used.
Whether the biggest difference between the above two methods has an impact on future generations. Germ cell gene therapy is a restricted area because it inherits genes to future generations, involves genetic and ethical issues, and current technical limitations. For example , the Ethical Guiding Principles for Human Embryonic Stem Cell Research, promulgated in 2003, may be used for genetic editing and modification of human embryos for research purposes, but the in vitro culture period may not exceed 14 days from the beginning of fertilization or nuclear transfer. Therefore, He Jiankui’s editing of the fetus in 2018 is prohibited by the law and should be handled in accordance with relevant Chinese laws and regulations. Therefore, under current conditions, somatic gene therapy has become the main treatment.
The development of gene therapy
The first successful case of gene therapy was the treatment of primary immunodeficiency disease by transplantation of genetically-corrected autologous hematopoietic stem cells, although demonstrating good therapeutic effects, malignant transformation and adenoviral vector testing due to insertional mutations in the integration vector The impact of adverse events such as patient death led to a downturn in gene therapy [3].
In recent years, with the optimization of viral vectors and the emergence of gene editing technology , gene therapy has reignited a new dawn, mainly due to the use of in vitro strategies (for X-linked spinal cord injury, adenosine deaminase deficiency and adrenal brain). Breakthrough success in clinical trials of white matter malnutrition and in vivo methods (type 2 Leber congenital blackness and type B hemophilia), such as the end of 2018, Editas Medicine (founded by Zhang Feng) announced that it was used Gene therapy (named: EDIT-101) for the treatment of Leber congenital sputum type 10 (LCA10) has been approved by the US FDA, and EDIT-101 is expected to be the world's first CRISPR therapy for use in humans. EDIT-101 is administered by subretinal injection, and the gene editing system is directly delivered to the photoreceptor cells to achieve therapeutic effects. It has been approved by the FDA to enter the clinic.
Since 2012 , Western countries have approved six gene therapies:
1) Gleiberella is an AAV-based familial lipoprotein lipase deficiency therapy that was approved by the European Medicines Agency (EMA) in 2012.
2) IMLYGIC is a genetically modified herpes simplex virus type 1 for topical treatment of unresectable lesions in patients with melanoma. It was approved by the US Food and Drug Administration in 2015.
3) Strimvelis is a gamma-retroviral-based therapy for the treatment of severe combined immunodeficiency (ADA-SCID) caused by adenosine deaminase deficiency and was approved by EMA in 2016.
4) In 2017, the US FDA approved three gene therapies. KYMRIAH and YESCARTA are CD19-directed genetically modified autologous CAR T cell immunotherapy; both are suitable for the treatment of non-Hodgkin's lymphoma, and KYMRIAH is also suitable for the treatment of acute lymphocytic leukemia.
5) LUXTURNA is an AAV-based gene therapy for the treatment of repolar dystrophy associated with the biallelic RPE65 mutation.
The figure below shows the number of gene therapy and clinical programs registered and developed globally between 1995 and 1818. It can be seen that the number of registrations has increased rapidly since 2013, indicating that gene therapy has entered a stage of rapid development.
Advantages of a new generation of gene editing technology <br> Compared with viral vectors in traditional genetic engineering, gene editing technology provides a precise "surgical scissors" for gene insertion, deletion and correction, which greatly improves the precision of gene editing. Sex and safety. Currently, the use of CRISPR/Cas9 gene editing tools has been widely used in clinical gene therapy applications, and the core three major gene editing companies (CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics) are also on the market. These companies are committed to the development of gene therapy products. Although still at an early stage of development, it opens the way for clinical transformation of gene therapy.
In the past year, gene therapy, especially in genome editing, has made a series of breakthroughs, and published data on a number of clinical trials, demonstrating the effectiveness and safety of gene therapy for a variety of serious human diseases. The first batch of gene therapy drugs in the United States also received FDA approval. With the support of governments and charities, scientists and clinicians working on basal, translational, and clinical research will continue to innovate and develop more efficient and safe technologies. The biotechnology and pharmaceutical industries are also increasingly entering the field of gene therapy, reflecting the maturity of this field and the need to promote clinical transformation of gene therapy. However, there are still many challenges in gene therapy, including addressing the genotoxicity of integrated gene delivery vectors or non-targeted genome editing, and how to increase the efficiency of expression or editing of transgenes to effective therapeutic levels, and to address the immune response of repeated drug delivery in vivo. Wait. Gene therapy offers a lasting cure for human disease, as evidenced by the success of research and clinical trials over the past few years, demonstrating that continuing to be optimistic and positive about gene therapy is expected to bring this therapy to the future.
references:
[1] Xavier M. Anguela and Katherine A. High. 2019. Entering the Modern Era of Gene Therapy. Annual Review of Medicine . Vol. 70:273-288
[2] Cox, DBT, Platt, RJ, & Zhang, F. (2015). Therapeutic genome editing: prospects and challenges. Nature Medicine , 21(2), 121-131.
[3] Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 118:3132–42
[4] Maeder, ML, & Gersbach, CA. (2016). Genome editing technologies for gene and cell therapy. Molecular Therapy .
Cancer and rare diseases are currently the most focused areas of gene therapy. According to Pharma projects' report in 2018 [1], the current gene therapy category mainly involves: cancer, rare genetic diseases, cardiovascular diseases, infectious diseases, etc. Cancer and rare diseases are the two most recent clinical studies in gene therapy. The figure below shows the number of clinical trial indications for gene therapy as of 2017.

Figure 1: Number of clinical trial indications for gene therapy by 2017 [1]
How is gene therapy classified?
Gene therapy in a broad sense refers to the method of transferring certain genetic material into a patient, expressing it in the body, and finally reaching a certain disease. Narrow gene therapy refers to the way in which genes that function normally function to correct or replace the original defective genes for therapeutic purposes.
At present, gene therapy is mainly divided into the following three categories, including classification according to treatment route, mode of operation and target cells.
First, according to the route of drug delivery can be divided into two kinds of in vivo and in vitro gene therapy (Figure 2):
1. In vivo ( in situ cell gene therapy): The treatment of a disease by directly delivering a therapeutic gene to a patient's disease site using a non-viral or viral vector (either viral-based and gene-editing-based). This method is convenient to operate and is the best choice for some cell types that cannot be cultured in vitro. However, due to the uncertainty of in vivo editing, many events such as random integration or off-target are unavoidable, and some viral vectors can cause life-threatening immune responses in the body.
2. Ex vivo ( ex vivo ) (the patient's cells are modified in vitro and then transplanted back) : directly take the patient's own diseased cells, and modify the diseased cells by gene introduction in vitro to enable high-efficiency expression of therapeutic proteins. It is then expanded in vitro and finally returned to the patient for the purpose of treating the disease. This method can not use immune cells such as immune rejection in the use of autologous cells, and can also screen highly efficient transduction and off-target cells in vitro to achieve efficient and safe gene therapy. However, due to the cumbersome steps, the shortcomings of cell viability are also limited.
One of the main distinguishing factors in gene therapy in vitro and in vivo is the choice of vector (Figure 3). As described above, the vector can be classified into an integrated vector and a non-integrated vector . So how to choose?
l When introducing genetic material into stem cells, the integration vector is preferred, so that the donor DNA will be integrated into the genome of the stem cell and will be replicated during cell division, so that the genetic material can be transmitted to all daughter cells. .
For in vivo gene therapy, it is usually targeted to cells after mitosis. In these cells, since they do not divide again, long-term expression can be achieved as long as the introduced foreign gene is stably present in the cell; the stable episome is sufficient to drive the periodic expression of the foreign gene.

Figure 2 In vitro and in vivo pathways for gene therapy [2]
Most current gene therapy strategies for genetic diseases focus on two vectors:
1) Lentiviral (L V ) vectors for in vitro gene transduction into hematopoietic and other stem cells : such as bluedird Bio; LentiGlobin is a lentiviral vector-based in vitro gene therapy that shows great potential, However, the semi-random vector integration method has the risk of carcinogenesis. At the same time, the expression components in the lentivirus will gradually silence during the long-term homing and self-renewal of hematopoietic stem cells, which will reduce the curative effect and may not achieve the goal of lifelong cure. And this method requires a lot of viruses, which means that the huge cost is high.
2) Adeno-associated virus (A AV ) vectors for in vivo gene transduction to post-mitotic cell types : Luxturna, such as Spark, is an approved hereditary retinopathy for the treatment of RPE65 gene defects in 2017. The normal RPE65 gene is carried by the AAV virus and injected into the retina. The normal RPE65 gene is not integrated into the DNA of human cells, but the normal RPE65 protein is synthesized in the nucleus, thereby triggering the light-conducting pathway and restoring normal visual function. .

Figure 3: The most commonly used gene therapy virus vector [1]
Second, according to the way of genetic operation, it is divided into two categories (Figure 4 ):
1. The gene enhancer (gene augmentation) and gene inactivation (gene suppressi on): to express normal products by introducing a foreign gene, thereby compensating for defective function of the gene or the like, in addition to abnormal gene not required; or specific blocking of certain genes Translation or transcription to achieve inhibition of the expression of certain abnormal genes.
2. Edit gene (Gen ome editing), divided into correct gene (gene correction), knockout (gene knockout) was added and the gene (gene a d dtion): techniques by site-directed integration of the exogenous gene at a specific location normally The point is replaced or inserted to correct the abnormal sequence of the defective gene and to repair it in situ without any other changes in the genome.

Figure 4: Gene therapy classified by genetic manipulation [1]
According to the different target cells, it can be divided into two types: somatic cells and germ cells:
1. somatic gene therapy (S omatic cell) refers to the transfer of the normal gene into a somatic cell, so that expression of the gene product for therapeutic purposes. This method generally involves introducing a foreign normal gene into a chromosome-specific gene locus in a target cell, replacing the abnormal gene with a healthy gene, thereby exerting a therapeutic effect, and also reducing the possibility of random insertion causing a new gene mutation. There are still great difficulties in the fixed integration of the genome.
2. The germ cell gene therapy (G erm cell) is directly introduced into the germ cells of a normal gene to correct the defective gene. Not only can genetic diseases be treated in the contemporary era, but new genes can be passed on to future generations of patients, and genetic diseases can be cured. However, gene therapy of germ cells involves more problems and more complicated techniques. Therefore, more somatic gene therapy is now used.
Whether the biggest difference between the above two methods has an impact on future generations. Germ cell gene therapy is a restricted area because it inherits genes to future generations, involves genetic and ethical issues, and current technical limitations. For example , the Ethical Guiding Principles for Human Embryonic Stem Cell Research, promulgated in 2003, may be used for genetic editing and modification of human embryos for research purposes, but the in vitro culture period may not exceed 14 days from the beginning of fertilization or nuclear transfer. Therefore, He Jiankui’s editing of the fetus in 2018 is prohibited by the law and should be handled in accordance with relevant Chinese laws and regulations. Therefore, under current conditions, somatic gene therapy has become the main treatment.
The development of gene therapy
The first successful case of gene therapy was the treatment of primary immunodeficiency disease by transplantation of genetically-corrected autologous hematopoietic stem cells, although demonstrating good therapeutic effects, malignant transformation and adenoviral vector testing due to insertional mutations in the integration vector The impact of adverse events such as patient death led to a downturn in gene therapy [3].
In recent years, with the optimization of viral vectors and the emergence of gene editing technology , gene therapy has reignited a new dawn, mainly due to the use of in vitro strategies (for X-linked spinal cord injury, adenosine deaminase deficiency and adrenal brain). Breakthrough success in clinical trials of white matter malnutrition and in vivo methods (type 2 Leber congenital blackness and type B hemophilia), such as the end of 2018, Editas Medicine (founded by Zhang Feng) announced that it was used Gene therapy (named: EDIT-101) for the treatment of Leber congenital sputum type 10 (LCA10) has been approved by the US FDA, and EDIT-101 is expected to be the world's first CRISPR therapy for use in humans. EDIT-101 is administered by subretinal injection, and the gene editing system is directly delivered to the photoreceptor cells to achieve therapeutic effects. It has been approved by the FDA to enter the clinic.
Since 2012 , Western countries have approved six gene therapies:
1) Gleiberella is an AAV-based familial lipoprotein lipase deficiency therapy that was approved by the European Medicines Agency (EMA) in 2012.
2) IMLYGIC is a genetically modified herpes simplex virus type 1 for topical treatment of unresectable lesions in patients with melanoma. It was approved by the US Food and Drug Administration in 2015.
3) Strimvelis is a gamma-retroviral-based therapy for the treatment of severe combined immunodeficiency (ADA-SCID) caused by adenosine deaminase deficiency and was approved by EMA in 2016.
4) In 2017, the US FDA approved three gene therapies. KYMRIAH and YESCARTA are CD19-directed genetically modified autologous CAR T cell immunotherapy; both are suitable for the treatment of non-Hodgkin's lymphoma, and KYMRIAH is also suitable for the treatment of acute lymphocytic leukemia.
5) LUXTURNA is an AAV-based gene therapy for the treatment of repolar dystrophy associated with the biallelic RPE65 mutation.
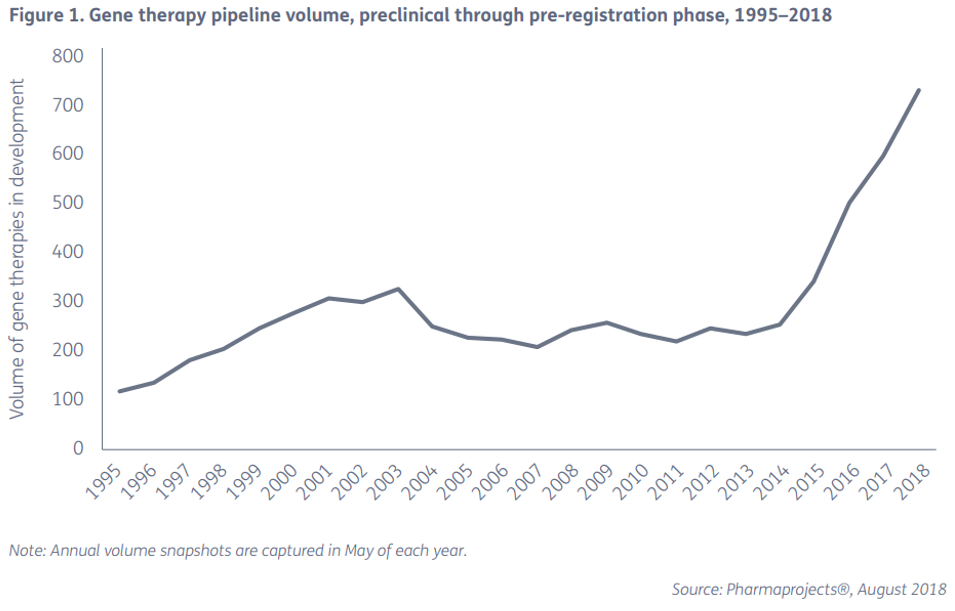
The figure below shows the number of gene therapy and clinical programs registered and developed globally between 1995 and 1818. It can be seen that the number of registrations has increased rapidly since 2013, indicating that gene therapy has entered a stage of rapid development.

Figure 5: Number of gene therapy and clinical projects registered and developed globally between 1995 and 1818
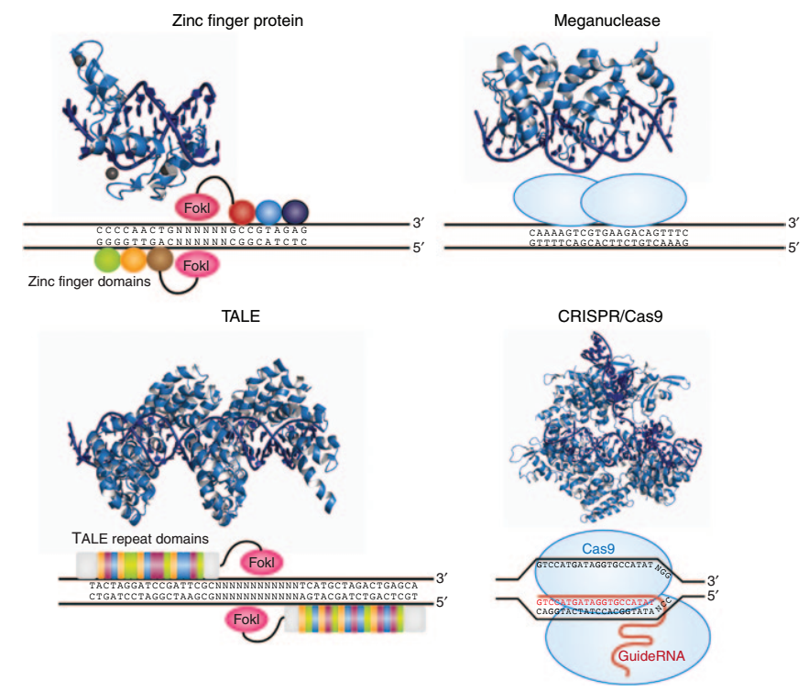
Breakthrough gene-editing technologies to promote the development of gene therapy <br> from the figure seen since 2013 the number of registered gene therapy straight up, largely thanks to the breakthrough gene editing technology, in particular the 2013 third-generation gene editing technology The CRISPR/Cas9 system was reported. The technical principle is that DNA double-strand breaks induce endogenous repair mechanisms of cells, and the precise introduction of DSB will facilitate the efficient integration of foreign genes. There are currently four systems that can accurately induce site-specific DSB: zinc finger nucleases (ZFNs), transcriptional activator-like effector nucleases (TALEN), meganucleases, and more recently the CRISPR/Cas9 system (Figure 6). . 
Figure 7: Four classical genome-edited DNA-targeting endonuclease systems [4]
Advantages of a new generation of gene editing technology <br> Compared with viral vectors in traditional genetic engineering, gene editing technology provides a precise "surgical scissors" for gene insertion, deletion and correction, which greatly improves the precision of gene editing. Sex and safety. Currently, the use of CRISPR/Cas9 gene editing tools has been widely used in clinical gene therapy applications, and the core three major gene editing companies (CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics) are also on the market. These companies are committed to the development of gene therapy products. Although still at an early stage of development, it opens the way for clinical transformation of gene therapy.
In the past year, gene therapy, especially in genome editing, has made a series of breakthroughs, and published data on a number of clinical trials, demonstrating the effectiveness and safety of gene therapy for a variety of serious human diseases. The first batch of gene therapy drugs in the United States also received FDA approval. With the support of governments and charities, scientists and clinicians working on basal, translational, and clinical research will continue to innovate and develop more efficient and safe technologies. The biotechnology and pharmaceutical industries are also increasingly entering the field of gene therapy, reflecting the maturity of this field and the need to promote clinical transformation of gene therapy. However, there are still many challenges in gene therapy, including addressing the genotoxicity of integrated gene delivery vectors or non-targeted genome editing, and how to increase the efficiency of expression or editing of transgenes to effective therapeutic levels, and to address the immune response of repeated drug delivery in vivo. Wait. Gene therapy offers a lasting cure for human disease, as evidenced by the success of research and clinical trials over the past few years, demonstrating that continuing to be optimistic and positive about gene therapy is expected to bring this therapy to the future.
references:
[1] Xavier M. Anguela and Katherine A. High. 2019. Entering the Modern Era of Gene Therapy. Annual Review of Medicine . Vol. 70:273-288
[2] Cox, DBT, Platt, RJ, & Zhang, F. (2015). Therapeutic genome editing: prospects and challenges. Nature Medicine , 21(2), 121-131.
[3] Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 118:3132–42
[4] Maeder, ML, & Gersbach, CA. (2016). Genome editing technologies for gene and cell therapy. Molecular Therapy .
Fresh apple is selected, trimmed, cut and dehydrated.
Apple dice,rings,Freeze-dried apples,dehydrated apples,freeze-dried diced apples
Xinghua Lvwei Foods Co.,Ltd , https://www.lvweifoods.com