Pharmaceutical Network October 17th Recently, Furen Pharmaceutical announced that the wholly-owned subsidiary Pharmaceutical Group has recently received the injection of recombinant human factor VIII-Fc fusion protein (250IU/bottle) approved by the State Food and Drug Administration. And two recombinant human factor VIII-Fc fusion proteins (1000 IU / bottle) for injection, "drug clinical trial approval", and will carry out clinical trials of the above products in the near future.
It is reported that the product has a corrective effect on coagulation dysfunction caused by human factor VIII deficiency, and is mainly used for preventing and treating hemorrhagic symptoms of hemophilia A and surgical bleeding treatment of such patients. At present, the drugs listed in China for the treatment of hemophilia A are coagulation factor VIII, recombinant human factor VIII for injection, and recombinant human factor VIIa for injection, of which only human factor VIII is a domestic product.
The domestically produced Class 1 new drug created by 100 million yuan has benefited from the priority review.
Figure 1: Timeline for review of recombinant human factor VIII-Fc fusion protein for injection (acceptance number: CXSL1700162)
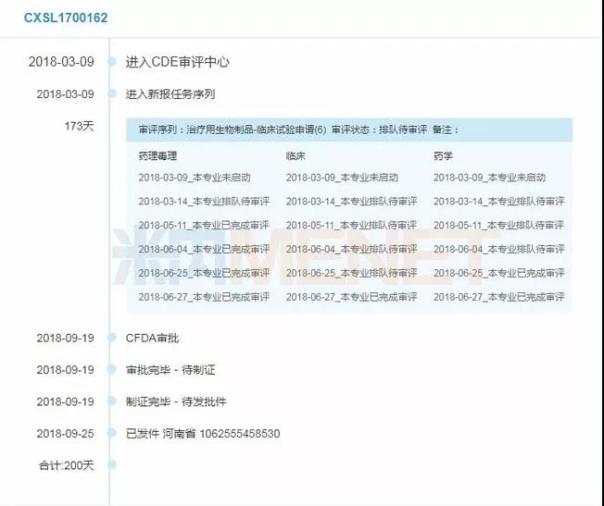
(Source: Mnet China MED China Drug Evaluation Database 2.0)
It is reported that the recombinant human factor VIII-Fc fusion protein for injection is mainly a new drug for the treatment of hemophilia A. The pharmacokinetic study in animals shows that the product has a longer half-life in vivo and provides long-term hemostasis. The potential to reduce the frequency of dosing. In addition, the product adopts Fc fusion technology, which has the potential to reduce immunogenicity and improve the safety of drug use. It has certain advantages over the domestically-listed varieties, and it is expected to meet the clinical needs of domestic coagulation factor VIII products after listing. The product entered the list of applications for registration of drugs in the 28th batch of priority review procedures of CDE. The Mnet China Drug Evaluation Database 2.0 data shows that the product has been used for 200 days from CDD to approval.
Furen Pharmaceutical's 2017 annual report data shows that the recombinant human factor VIII-Fc fusion protein project for injection belongs to one of the key biopharmaceutical research and development projects of the subsidiary Kailuan Group. As of the end of 2017, the accumulated research and development investment was 106,098,400 yuan.
After 7.8 billion "snake swallows", net profit from tens of millions to nearly 400 million
Figure 1: Performance of Furen Pharmaceutical in 2015-2018 (Unit: 100 million yuan)
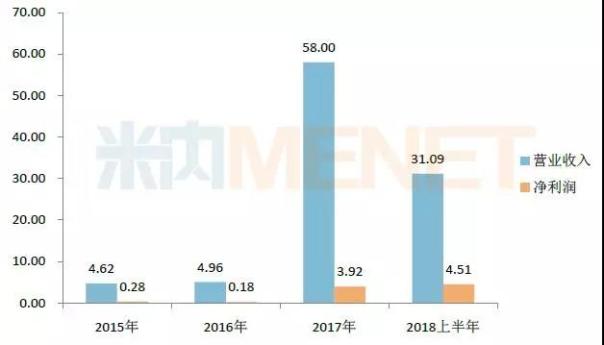
(Source: listed company annual report)
Subject to the small scale and single product, in recent years, the development of Chinese medicine business of Furen Pharmaceutical has fallen into a bottleneck, and sales scale and business performance are difficult to further improve in the short term. In December 2015, the “Proposal on the Company's Issuance of Shares and Payment of Cash to Purchase Assets and Raise Matching Funds and Related Party Transaction Plan†was approved by the Board of Directors. On December 25, 2017, the approval of the China Securities Regulatory Commission was approved. The pharmaceutical industry issues shares to all shareholders of Kaifeng Pharmaceutical and pays cash to purchase assets and raise matching funds. On December 26, 2017, the original shareholders of Kaifeng Pharmaceutical transferred their equity to Furen Pharmaceutical and completed the asset delivery procedures.
It is reported that in the above-mentioned trading plan, Furen Pharmaceutical purchased 100% of its holdings of the Pharmaceutical Group from 14 counterparties such as Furen Group, and the target assets were valued at RMB 789.90 million. At the same time, no more than 10 eligible non-public offerings will be used to raise funds for the non-public offering of shares, and the total amount of funds raised will not exceed RMB 530 million. Furen Pharmaceutical acquired the Biomedical R&D company of Furen Group through Kailuan Group to build a platform for large-scale comprehensive pharmaceutical listed companies covering chemical, Chinese patent, API and biopharmaceuticals.
According to the annual report data, after the consolidated statements in 2017, the operating income of Furen Pharmaceuticals rose to 5.8 billion yuan, and the net profit increased from about 18 million yuan in 2016 to 392 million yuan. The overall performance of the company has been greatly improved.
   "Gorgeous transformation" Furen Pharmaceutical antibiotics annual sales of 2 billion
Prior to the transformation, the main production and operation of Furen Pharmaceutical was the subsidiary of Henan Furentang Pharmaceutical Co., Ltd. The main product was proprietary Chinese medicine. The Pharmaceutical Group is a modern large-scale pharmaceutical group that integrates the research, development, production and sales of chemical drugs, proprietary Chinese medicines and APIs. Currently, it has more than 480 drug approval numbers, including the “Medical Insurance Catalogue (2017 Edition)â€. More than 300.
Table 1: Revenue of various therapeutic areas of Fu Jen Pharmaceutical in 2017 (Unit: 10,000 yuan)
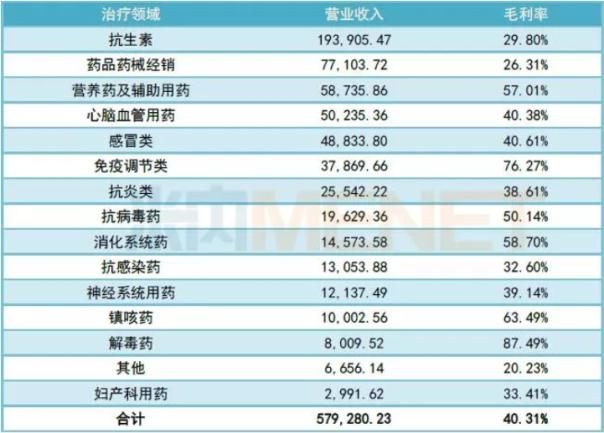
(Source: listed company annual report)
After the pharmaceutical group was injected into Furen Pharmaceutical, the company has become a large-scale comprehensive pharmaceutical listed company platform covering chemical drugs, proprietary Chinese medicines, APIs and biopharmaceuticals. In 2017, Furen Pharmaceutical's business income exceeding 500 million yuan included antibiotics, pharmaceutical and pharmaceutical distribution, nutraceuticals and auxiliary drugs, and cardiovascular and cerebrovascular drugs. The operating income of antibiotics in 2017 was close to 2 billion yuan.
The company's leading products are ceftriaxone sodium for injection, cefoperazone for injection, amikacin sulfate injection, doxycycline hydrochloride (raw drug), compound licorice tablet, psoralen injection, antiviral oral solution , Lentinus edodes polysaccharide tablets, Xiangdan injection, hypoxanthine tablets, Shengmaiyin oral solution, adenosine monophosphate for injection, tooth pain Xiaoyanling granules, Xiaoer Qingreanning granules, Shenqijianwei granules, lipid-lowering Laxative capsules, sugar urinary capsules, donkey-hide gelatin, antler gum and other products.
Conclusion
After the transformation, Furen Pharmaceutical has established Zhengzhou technology research and development and international cooperation platform, Beijing innovation research and development platform, Shanghai research and development platform, etc., and vigorously develop new product research and development. At present, the company's key biopharmaceutical research and development project, the recombinant human factor VIII-Fc fusion protein for injection has been successfully approved. In 2018, the company will continue to develop diabetes products, ceftaroline ester bulk drugs and powder injection projects. , psoralen gel project, Ai Kuiling oral liquid project, aprepitant bulk drug and capsule project, refined glutamic acid and (small volume) injection project.
   Source: Listed company announcement
Led Facial Mask,Led Light Facial Mask,Led Therapy Facial Mask,Led Facial Skin Care Mask
Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizons.com