Extraction of phospholipids from plasma using Ostro sample preparation products
Mark Ritchie, Mainak Mal and Stephen Wong
Waters Corporation, Singapore
Introduction:
The common goal of most "omics" studies is to identify disease molecular markers, understand the mechanisms of disease action, and discover potential drug targets. Normally, body fluids used for research are very sensitive to specific pathological conditions due to close contact with living organs and tissues. In lipidomics analysis, plasma phospholipids are usually studied by liquid-liquid extraction. There have been applications for solid phase extraction in the past. Aminopropyl silica gel columns can also be used to analyze lipids, but focus on fatty acids or screen depending on the elution conditions. The Ostro 96-well sample preparation plate is designed to study small molecules in daily bioanalysis to capture and remove large amounts of phospholipids during sample preparation. In this general procedure, lipids are retained on the Ostro plate and are not eluted. By adjusting the loading and elution conditions, selective enrichment of specific types of phospholipids or extraction of all phospholipids can be achieved.
Experimental conditions:
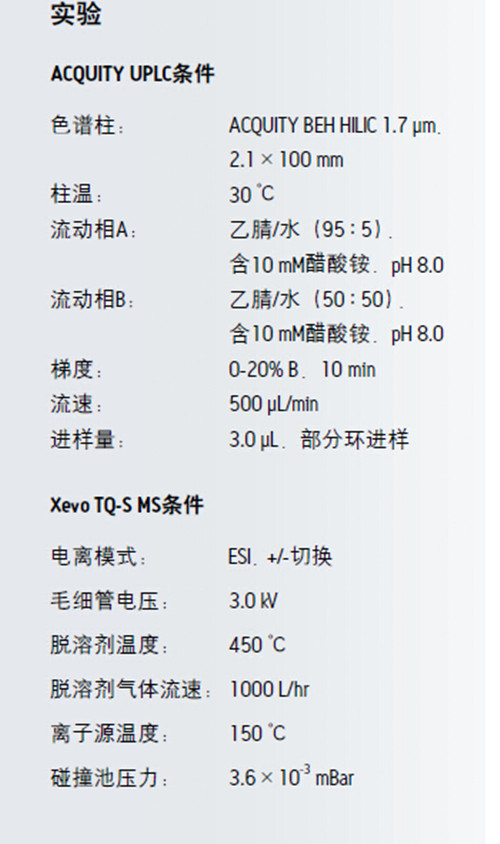
Sample Preparation:
Sources of human plasma samples: volunteers from the National University of Singapore Biosciences, or commercial samples (Sigma, P/N P9523).
Extraction process:
1. Place the Ostro plate on a 2 mL collection plate and place it on the vacuum unit.
2. Pipette 100 μL of plasma directly into the Ostro plate using a pipette.
3. Add 800 μL of ethanol to the well and pipette 10 times to ensure the sample is well mixed.
4. After mixing, apply 15 Ì‹ Hg vacuum until the solvent is completely vented (about 1 minute).
If there is no liquid flowing out under 15 Ì‹ Hg, a higher vacuum condition can be used.
5. Repeat steps 3 and 4 and mark the collected traffic as the “flow through†section.
6. Remove the collection plate containing the flow-through portion from the vacuum unit and reinstall the new plate for collection of the elution portion.
7. Add 800 μL of elution solvent (4.5:4.5:1/chloroform:methanol:triethylamine) to the well.
8. Apply 15 Ì‹ Hg vacuum until the solvent is completely vented (about 1 minute).
9. Repeat steps 6 and 7 and mark the collected volume as the “elution†section.
10. Dry the flow through and elute sections (using a nitrogen evaporator for approximately 1.5 hours).
11. Re-dissolve (E) and flow through (FT) fractions with 200 μL of 1:1 (v/v) chloroform:methanol.
Note: Be careful to ensure that the tip is placed in the designated position on the sample plate to avoid sample loss or mixing.
For comparison, equal amounts of human plasma were extracted using Bligh-Dyer method 1. Given the wide range of dynamic differences between different phospholipid species, we analyzed two concentrations of the sample: undiluted, diluted 1/100 with acetonitrile. The multi-reaction monitoring method with positive and negative switching was used, and the multi-reaction monitoring time setting was consistent with the elution time of the specific type of phospholipid 1. A brief introduction of the method is shown in Table 1.
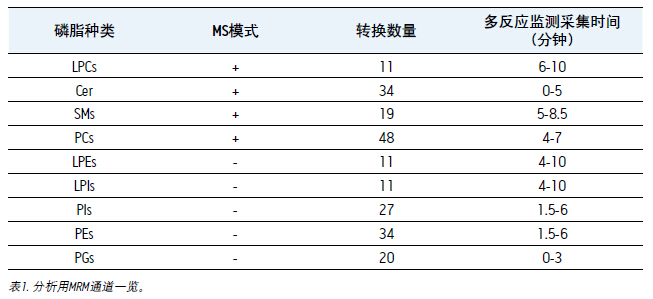
The signals obtained for each component are automatically integrated using the pre-programmed TargetLynxTM method. The average peak area threshold is set to 1000.
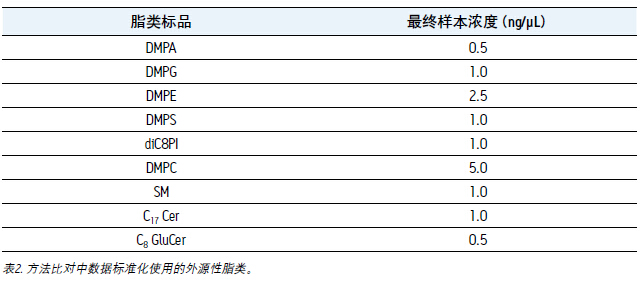
Before the analysis, the exogenous lipid internal standard was added to the sample as a blank control, and the two sample preparation methods were compared. This step is to normalize the data before calculating the relative extraction rate of the method or elution component. The lipids and concentrations added are shown in Table 2.
Results and discussion:
Comparison of extraction rates using Ostro sample preparation and Bligh-Dyer method
The pooled volunteer plasma was extracted, two each. Since the peak area threshold is set to 1000, in order to detect lipids, the peak area of ​​the MRM channel is at least greater than this threshold. The peak area values ​​of each lipid were normalized using a ratio of exogenous phospholipids of the same type to which the post-extraction was spiked. Subsequently, the same extract of the same method was used to calculate an average distribution value for these ratios so that each part of the lipids could obtain a unique standard value. The resulting lipid values ​​of the Ostro flow-through and elution fractions were removed to obtain the same lipid-derived data from the Bligh-Dyer method, and the result was the extraction rate of each lipid. The average extraction rate of each lipid is shown in Table 3.
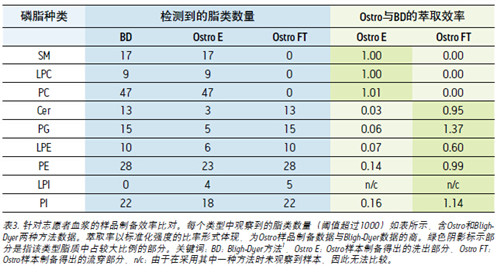
Sphingomyelin (SMs), phosphatidylcholine (PCs), and lysophosphatidylcholine (LPCs) are all completely retained by the Ostro plate and appear only in the eluate. The same phospholipids have the same strength in both extraction methods.
Ceramide (Cer) and phosphatidylglycerol (PGs) have almost no affinity under the same conditions, mainly in the flow-through portion, and only a very small portion is retained and appears in the eluted portion. Qualitatively the same PG lipids, the extraction rate using the Ostro plate is larger than that of the Bligih-Dyer method. Phosphatidylethanolamine (PEs) and phosphatidylinositol (PIs) performed consistently, and the extraction rate was consistent with the Bligh-Dyer method on the Ostro plate, but remained stronger on the Ostro plate and slightly higher in the eluate. The same hemolytic PE (LPE) class was found in both extraction methods, but the recovery using the Ostro plate was only 60% of the Bligh-Dyer method. On the other hand, the use of Ostro sample preparation greatly promoted the extraction rate of lysophosphatidylinositol (LPIs), and five kinds of lipid species were observed using the Ostro plate than the Bligh-Dyer method. Most LPIs (90%) are not retained on the Ostro board. In general, SMs, PCs and LPCs can be retained on the Ostro plate, and the elution solution can only be chloroform: methanol: triethylamine (4.5: 4.5:1). Other types of phospholipids did not significantly retain the plate under these conditions and passed through the plate and appeared in the flow-through portion.
Repeatability of Ostro phospholipid extraction method
Fourteen samples of commercially available human plasma were extracted using Ostro. The samples of the three extracts of each phospholipid were analyzed in the flow through (FT) and elution (E) portions. The relative standard deviation percentage (%RSDs) of the peak area of ​​each lipid in all replicates was calculated as shown in Table 4.
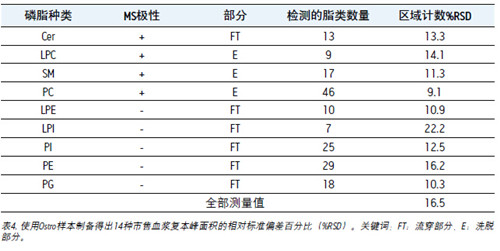
The %RSD shown above is affected by the ionic strength, and the fewer types produce more errors in this experiment. In the case of the same threshold, the error of the analysis (LC-MS) method is within 5%.
in conclusion:
The Ostro sample preparation method for phospholipid extraction is simple and reproducible. The number and amount of phospholipids detected in plasma using this method is better than the standard extraction method (Bligh-Dyer), and Ostro plates have significant advantages in the recovery of LPIs and PGs. This method is suitable for small and large lipidomics studies. Since the Ostro plate is 96-well and requires no centrifugation, it can also be used for laboratory automation that cannot be achieved by liquid-liquid extraction. Compared to liquid-liquid extraction, the Ostro sample preparation method also significantly reduces the amount of solvent used. The method can be applied to both the extraction of all phospholipids and the selective extraction of specific phospholipids. For example, by analyzing the flow-through portion, it is possible to study a lower content of some types of phospholipids without being affected by the linear range or ion suppression of excessively large amounts of PCs.
references:
1. Bligh, EG and Dyer, WJ; Canad.J. Biochem. Physiol (1959) 27, 911-917
2. Zhao, Z and Xu, Y; J. Lipid Res (2010) 51, 652-659
3. Burdge et al.; British Journal of Nutrition (2000), 84, 781-787
4. Kim et al.; J. Lipid Res (1990), 31, 2285-2289
5. UPLC BEH HILIC: “T he Preferred Separation Chemistry for Targeted Analysis of
Phospholipids", Mark Ritchie, Mainak Mal, and Stephen Wong. Waters Application Note
No. 720004219EN, January 2012.
Canned Baby Corn Whole,Baby Corn Whole Canned,Baby Corn Whole Tin,Corn Whole Canned
ZHANGZHOU TAN CO. LTD. , https://www.zztancan.com