The first domestic PD-1 monoclonal antibody drug enters clinical phase II
November 24, 2016 Source: Biology
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Jiangsu Hengrui Pharmaceutical Co., Ltd. and its holding subsidiary Shanghai Hengrui Pharmaceutical Co., Ltd. recently launched Phase II and III clinical trials of SHR-1210 for injection:
1. Basic information of clinical trial approvals
Drug Name: SHR-1210 for Injection
Dosage form: Injection specification: 200mg Application stage: Clinical applicant: Jiangsu Hengrui Pharmaceutical Co., Ltd.; Shanghai Hengrui Pharmaceutical Co., Ltd. Acceptance number: CXSL1400153 Su approval number: 2016L01455 Review conclusion: According to the Drug Administration Law of the People's Republic of China and Relevant regulations, after review, this product meets the relevant requirements for drug registration, and approves this product for clinical trials.
2. Other relevant information on drugs On December 29, 2014, the company and its holding subsidiary, Shanghai Hengrui Pharmaceutical Co., Ltd. submitted a clinical registration application to the State Food and Drug Administration and was accepted. The main indication for this drug is solid tumor. Programmed Death Receptor 1 (PD-1), a protein receptor expressed on the surface of T cells discovered in 1992, is involved in the process of cell apoptosis and is a very potential new target in the field of tumor immunity.
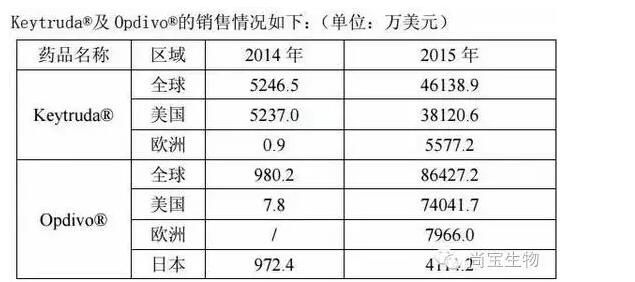
Currently marketed overseas anti-PD-1 monoclonal antibody products are two trade names for the Keytruda ® and Opdivo ®.
Keytruda ® was developed by Merck and was first approved by the FDA in September 2014 with a specification of 50 mg/vial for the treatment of melanoma and NSCLC.
Opdivo ® was developed in collaboration with Ono Pharmaceuticals and Bristol-Myers Squibb. It was first approved in Japan in July 2014 and is available in 20mg/bottle and 100mg/bottle for the treatment of melanoma and NSCLC. The two drugs are the first anti-PD-1 monoclonal antibody products approved by the FDA and Japan.
According to the data provided by the website of the State Food and Drug Administration and the Drug Evaluation Center, there is currently no anti-PD-1 monoclonal antibody approved for production in China. Bristol-Myers Squibb has submitted eight clinical applications for the import of Nivolumab injection, and some have received clinical approvals. Merck submitted nine clinical applications for the import of Pembrolizumab injection, which have been “certified and issuedâ€. Among the domestic enterprises, Taizhou Junshi Biotechnology Co., Ltd., Baekje Shenzhou Biotechnology Co., Ltd. and Hengrui Medicine have obtained recombinant humanized anti-PD-1 monoclonal antibody injection and BGB-A317 injection respectively. Clinical approval for SHR-1210 for injection.
In 2015, the company licensed the drug to Incyte, Inc., Inc., Inc. will receive exclusive global clinical development and marketing rights in addition to mainland China, Hong Kong, Macau and Taiwan. At present, Incyte has completed dose escalation trials in Phase I clinical trials in Australia and is based on this assessment, suspended into the group, and then decided to proceed to the next clinical work.
Up to now, the company has invested R&D expenses of approximately RMB 23.31 million in the SHR-1210 R&D project for injection.
PD-1 monoclonal antibody, which is the most important product for future anti-tumor drugs, has been listed abroad but has not yet been approved in China. At present, among the domestic enterprises, Hengrui Medicine, Junshi Bio and Baekje Shenzhou have obtained the clinical approval of Anti-PD-1 monoclonal antibody as the first echelon, and it is expected to compete with foreign companies in the future, Opdivo and Keytruda.
The company is the first to enter the clinical phase 2, and is expected to seize the opportunities in the future competition. It is also worth noting that the status of Incyte's project is suspended, but the factors may be multi-faceted and have not affected Hengrui's clinical trial of SHR-1210 in China. Hengrui Medicine has achieved a virtuous circle of development and has sufficient strength to support its research and development of new drugs. In the future, Hengrui will continue to strengthen its leading position in the fields of anti-tumor and diabetes.
The raw materials of health care products should mainly be plant extracts, which have the commonness of general food and can regulate the function of human body. It is suitable for specific people to eat, but not for the purpose of curing diseases.
AMULYN is a brand that dedicated to the production, development and sales of Natural Plant Extracts, Plant-based Ingredients, Natural Pigment , Natural Sweetener, Fruit and Vegetable Powder, Probiotics Powder and Health Ingredients products.
Propolis Extract,Phosphatidylserine,Inulin,Spirulina Powder,Coconut Oil MCT Powder
Xi'an Gawen Biotechnology Co., Ltd , https://www.amulyn-bio.com