Among the current cancer-targeted drugs, small molecules or monoclonal antibody inhibitors against oncogenic proteins are dominant. However, over time, many cancer cells become resistant to such drugs, such as generating new mutations or activating other oncogenic proteins. Over the past 15 years, some researchers have targeted the “cleaners†of the cell, the ubiquitin-proteasome system, when looking for other anticancer pathways. The ubiquitin-proteasome system is responsible for cleaning up unwanted or harmful proteins in cells. By activating this system-specific cleanup of oncogenic proteins, it is expected to restore protein homeostasis and prevent cancer. Recently, Science published a long article, reviewing and looking forward to the development of anticancer drugs targeting ubiquitin-proteasome.
A new model of anticancer drugs: induced protein degradation
Many cancer-targeted new drugs target oncogenic proteins. These small molecule drugs usually bind to the active sites of those oncogenic proteins to inhibit their activity. For this mode of drug, continued binding to the active site of the protein is necessary to maintain its efficacy. Many of these oncogenic proteins are kinases with structurally similar active sites, and the use of high doses of small molecule drugs is likely to cause non-specific side effects.
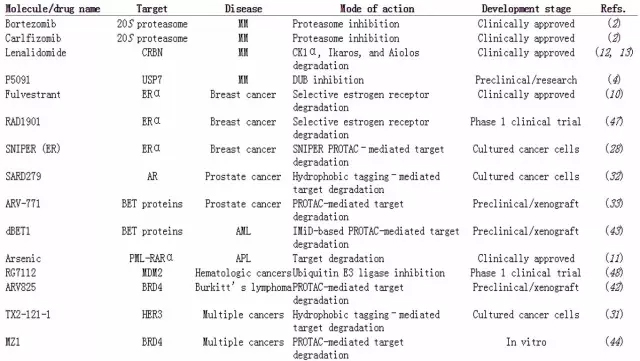
â–²Anticancer drugs acting on the mechanism of protein degradation (Source: Science)
Induced protein degradation is a new anti-cancer mechanism that has recently emerged. In theory, only small molecule drugs need to be combined with oncogenic proteins for a short time, and the oncogenic protein should be labeled as “need to clean upâ€. These drugs do not require high concentrations, can be recycled, and proteins need to be re-synthesized to restore function after degradation, which greatly delays the development of resistance.
The ubiquitin-proteasome system degrades the protein into several steps. The E3 ligase first binds the target protein with a ubiquitin protein tag, and then the protein undergoes multiple rounds of ubiquitination with multiple ubiquitin tags. The protein after polyubiquitination is recognized by the 26S proteasome and is degraded.
Small molecule drugs that simultaneously inhibit protein activity and promote protein degradation
Studies have shown that some small molecule drugs used to inhibit the activity of oncogenic proteins can also promote the degradation of target proteins. For example, canertinib is an inhibitor of the tyrosine kinase ErbB-2, and ErbB-2 is expressed in a variety of cancers, while canertinib can increase the polyubiquitination of ErbB-2 to promote its degradation. The fulvestrant, an inhibitor of ERα, is another example that also promotes the degradation of ERa. In breast cancer patients with ERa, fulvestrant is more effective than another ERα inhibitor, tamoxifen, which does not promote protein degradation. The arsenic trioxide, a very effective drug for acute promyelocytic leukemia, can also promote the degradation of the fusion oncogenic protein PML-RARα, which is common in patients with this disease.
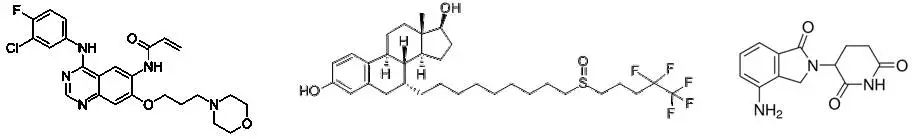
â–²Canertinib (left), fulvestrant (middle) and lenalidomide (right) molecular structure
Sugar Free Sweetener,Low Sugar Sweetener,Rare Sugar,Low Calorie Sugar,Health Sweetener
Shandong Bailong Chuangyuan Bio-tech Co.,Ltd. Qingdao Branch , https://www.sdblcycn.com