Liquid biopsy
June 27, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];As an alternative to traditional biopsy and a new technology for early screening of cancer, liquid biopsy technology is a representative diagnostic technique in the field of “precise medical†by obtaining tumor information through non-invasive blood sampling to assist cancer treatment.
At present, the main biomarkers derived from liquid biopsy are circulating tumor cells (CTC), circulating tumor DNA (ctDNA), exosomes and circulating RNA. These substances can enter the blood circulation through the blood vessels around the tumor, and these substances can be detected by taking blood samples, which can be used for early diagnosis of tumors, therapy selection, prognosis judgment and therapeutic effect monitoring.
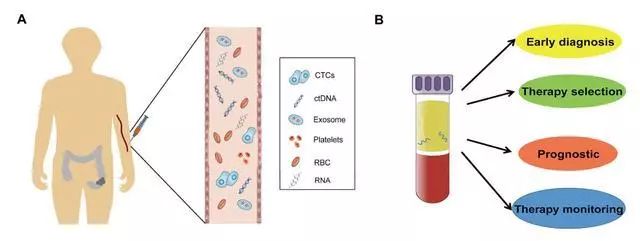
Figure: The content and role of liquid biopsy
1. Circulating Tumor Cells (CTCs)
In 1869, Australian doctor Ashworth first proposed the concept of Circulating Tumor Cells (CTCs). Currently, CTCs are collectively referred to as various types of tumor cells present in peripheral blood.
The necessary stages in the transfer of CTCs:
CTCs migrate to the blood vessels from the tumor tissue, enter the blood circulation through the blood vessels, overcome the killing effects of various factors in the blood and blood circulation, and finally form a new metastatic lesion and other physiological steps, so that the CTCs can be smoothly invaded and transferred. Thereby promoting the progress of related diseases.
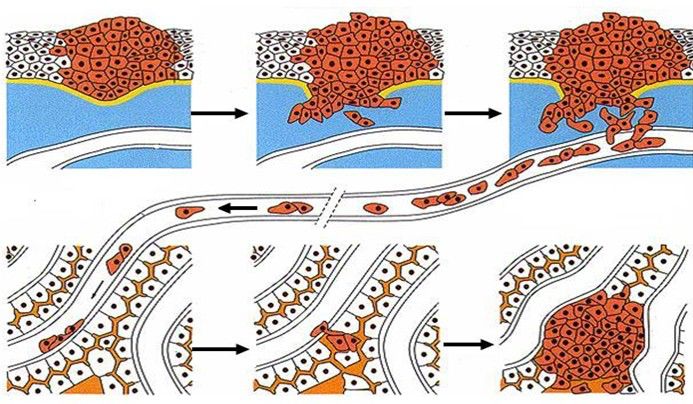
Figure: CTCs transfer process
Detection of CTCs:
The detection of CTCs, including cell enrichment and cell detection, can be combined in different ways to form different techniques. The most common method of enrichment is antibody-mediated or physical enrichment followed by immunohistochemical counting or genetic analysis.
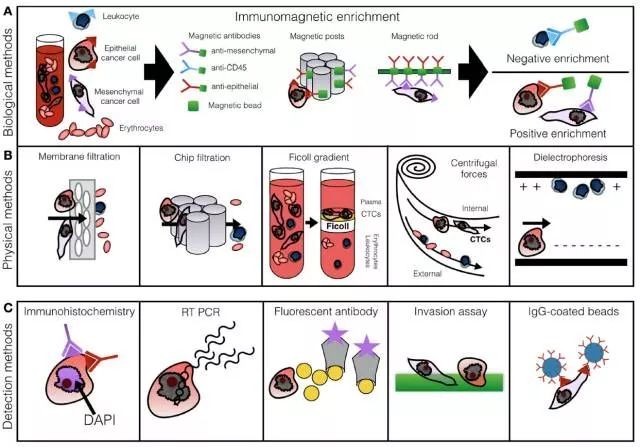
Figure: CTCs cell enrichment and cell detection procedures (line A shows the magnetic bead-antibody enrichment method; line B shows the physical enrichment method; line C shows the most commonly used cell detection method.)
Tumor lesion information can be obtained by the amount of CTC in the blood as well as protein expression and sequence detection.
A generation of CTC technology has been developed for the detection of CTC quantities.
Immunicon's Cellsearch CTC testing system passed FDA certification in 2004 and is the only CTC testing system approved by the FDA for clinical use. It is arguably the gold standard in the industry. In 2012, the Cellserach system entered the Chinese market through CFDA certification. Immunicon is currently acquired by Johnson & Johnson's subsidiary Veridex. Although the first generation of CTC technology is leading the clinical application, the current technical level is still not perfect. It can only be used to detect the number of CTCs, and it is impossible to carry out further in-depth testing. In addition, the sensitivity is low, and the application is limited to metastatic breast cancer and metastasis. Colorectal cancer and metastatic prostate cancer.
The second-generation CTC technology is accelerating the development of R&D. Compared with the first generation, the second-generation CTC will be able to achieve more comprehensive detection of gene sequencing, protein expression, and chromosomal variation levels in addition to the number of CTC cells, and obtain more tumor information. Sensitivity will be further improved to achieve early detection and treatment of tumors. The second-generation CTC has a large number of participating companies, but there are no products that have been approved for listing. There are no industry standards, no industry giants appear, and there are more participants in the competition and the technology is maturing.
Second, plasma free tumor DNA ('Cell-Free' Circulating Tumor DNA, ctDNA)
Circulating tumor DNA (ctDNA) is a DNA fragment of a tumor gene that is constantly flowing in the human blood circulation system and carries certain characteristics (including mutation, deletion, insertion, rearrangement, copy number abnormality, methylation, etc.). Generally, tumor cells derived from necrosis or apoptosis, circulating tumor cells and exosomes secreted by tumor cells. Tumor-specific mutation information can be obtained by sequencing and capturing ctDNA fragments, helping doctors diagnose and personalize medication guidance.
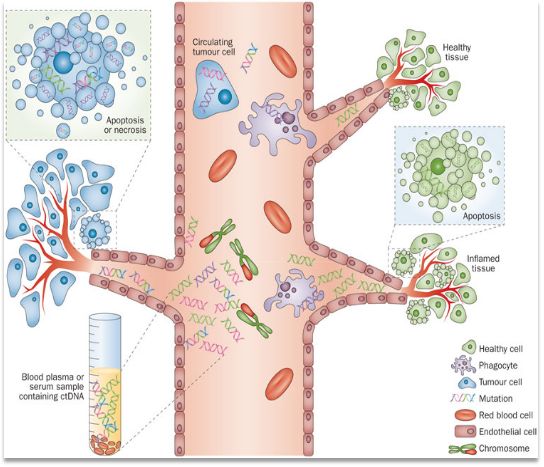
Figure: ctDNA source
Detection of ctDNA:
The detection platform of ctDNA currently has second-generation gene sequencing and digital PCR, which can detect the sequence information and sequence number of ctDNA, and is suitable for common tumors such as non-small cell lung cancer, breast cancer, colorectal cancer and skin cancer. Most of the genes are mainly KRAS, PTEN, EGFR, BRAF and other popular genes. Fragment capture of ctDNA requires prior knowledge and preparation of the mutation to be detected, and the preparation of the mutant library is cumbersome and costly. There are no FDA-certified products in ctDNA, and it is still in the research stage. Compared with CTC, both are liquid biopsy, high sensitivity and low cost, suitable for most tumor detection, and can be extracted from blood at the same time to obtain complementary information.
Third, exosomes (exosome)
Exosome is a 30-150nm small vesicle secreted by cells containing complex RNA and protein. It is mainly derived from multivesicular bodies formed by intracellular lysosomal microsomes. The membrane is fused to the cell membrane and released into the extracellular matrix.
Exosomes found in sheep reticulocytes in 1983 have become stars in the research field in recent years, and a large number of related studies have been reported. In particular, since 2015, the research and application of exosomes has developed rapidly, and the number of projects and articles published by the National Natural Science Foundation of China has increased significantly.
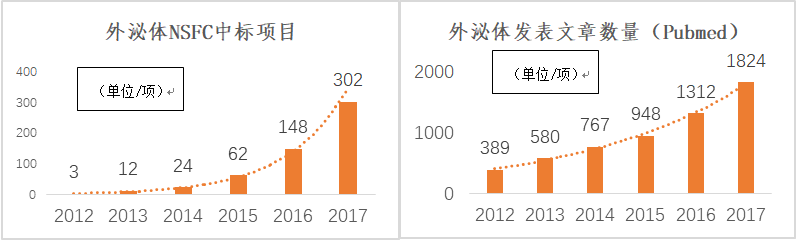
Figure: The National Natural Science Foundation of the Exosomes Wins the Project
Exosomes and tumor development
Exosomes are important players in tumor diagnosis, tumor development, and cancer treatment.
The main function of exosomes is to deliver a variety of biomolecules, including proteins, peptide ligands, DNA and RNAs. Therefore, the exocrine secreted by tumor cells plays a role in tumor development and treatment mainly in the following aspects: 1. Promoting tumor cell development, invasion and metastasis; 2. Promoting tumor angiogenesis; 3. Tumor immune regulation; 4. Tumor Chemosensitization.
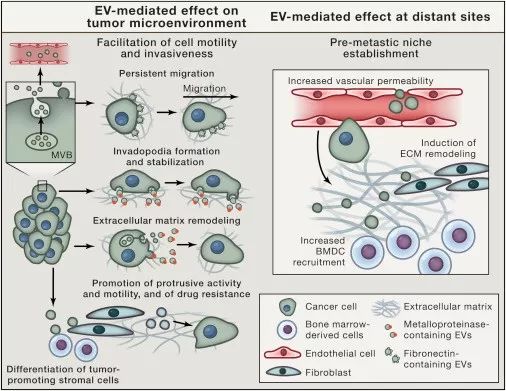
Figure: The role of exosomes in tumor development
Exosomes analysis
The exosomal database ExoCarta currently contains 9769 proteins, 4946 mRNA entries, 3408 mRNAs, 2838 miRNAs, and 1116 lipid entries.
After extracting exosomes by ultracentrifugation or magnetic beading, the exosome isolated and purified can be used as protein or RNA (microRNA and LncRNA), and 36% of researchers use Western blot or other methods to identify proteins, 29% The microRNA expression profile was analyzed by qPCR and 9% microarray expression profiling was performed using a chip. At the same time, there are many users of next-generation sequencing, 13% of them use NGS to conduct microRNA/small RNA research, 9% conduct mRNA research, and mass spectrometry based on omics research is more and more commonly used. In addition, most of the current work is still in the research stage, and there are not many biomarkers developed and validated, and there are fewer developments in diagnosis and therapy. Razvi believes that the qualitative and quantitative research of exosome is a fast-growing market. At the same time, there are opportunities and challenges in the market for circulating biomarkers. However, the success of non-invasive prenatal testing technology has led to the belief that circulating biomarkers are expected to play a role in the diagnosis of tumors and other diseases.
Liquid biopsy technology makes screening in the early stages of cancer possible. Whether it is in the field of tumor diagnosis, treatment or monitoring, the advantages of liquid biopsy make it the most non-invasive diagnostic tool for tumors with high clinical value and market. prospect.
The entire liquid biopsy field can be broadly divided into upstream sample capture technology, midstream sequencing services and downstream data analysis. Upstream technology and downstream data analysis are the links of higher technical barriers. The current R&D companies are also focusing on upstream technology development, and data accumulation is a follow-up link. Gene sequencing is a relatively mature link. Companies that provide sequencing services in China, such as Huada Gene and Daan Diagnostics, have already occupied a certain market, and sequencing technology is constantly being updated.
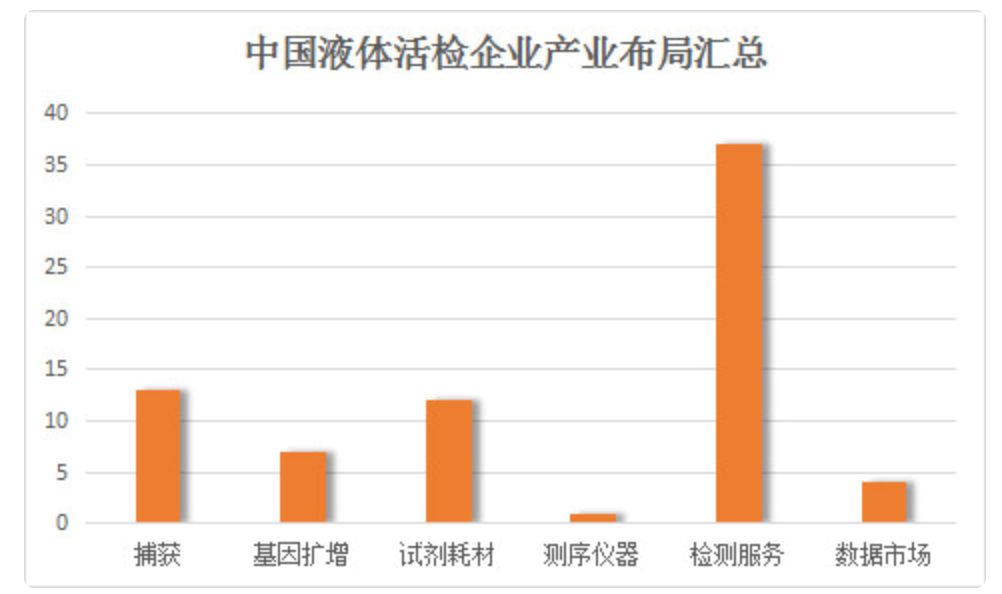
Figure: Summary of industrial layout of Chinese liquid biopsy enterprises (data source: arterial network)
According to the network layout of domestic liquid biopsy-related enterprises in the middle of 2017, the liquid biopsy can be divided into six sub-areas, namely upstream capture, gene amplification, reagent and instrument market; midstream detection service market and downstream data market.
Due to the technical difficulty of the upstream market, more companies choose to lay out in the middle reaches. At present, there are 13 companies involved in the upstream capture market, 6 companies involved in gene amplification, and 37 companies in the midstream.
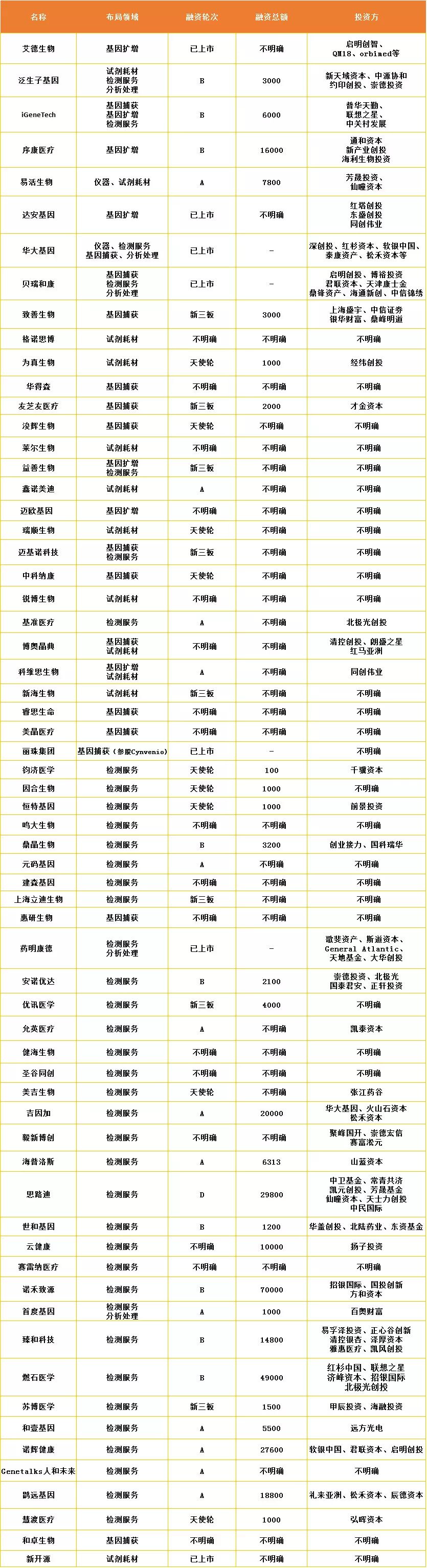
Table: Liquid biopsy-related business scans (data source: arterial network)
Dried Garlic Flakes,Fried Garlic Flakes,Slice Of Garlic,Roasted Garlic Slices
shandong changrong international trade co.,ltd. , https://www.changronggarliccn.com