Release date: 2016-10-14
In March of this year, Tiangong Society reported that a research team led by Hannifer A. Lewis, a professor of bioengineering at Harvard University's Hansrg Wyss, invented a method that could use human stem cells, extracellular matrices, and vascular endothelial cells. The circulation channel 3D printed a thick vascularized tissue structure and maintained activity for more than a month. Now that the team goes one step further, 3D creatures have printed a tubular 3D kidney structure that reproduces the function of the kidneys and takes a step closer to bioprinting functional human tissues and organs.
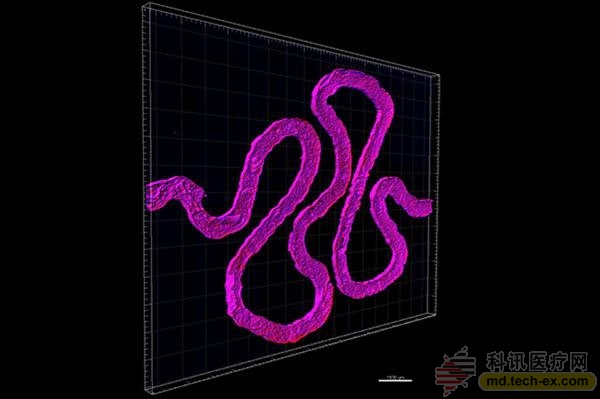
Working closely with Roche Pharmaceuticals scientist Annie Moisan, they built a functional 3D kidney structure based on the previous structure that contains living human epithelial cells that make up the surface of the renal tubules. The study is currently published online in the journal Scientific Reports.
“The current work further extends our bioprinting platform to create a functional human tissue structure that is both technical and clinical,†said Professor Lewis.
It is understood that the 3D kidney structure created by the Lewis team simulates the proximal tubule, the longest and thickest segment of the tubule, and is an important component of each nephron. The so-called nephron is responsible for the key function of switching between blood and urine. Each side of the kidney has 100-1.5 million nephrons. At the curl of the proximal tubules, approximately 65-80% of the nutrients are reabsorbed from the kidney filtrate and transported back to the blood. Therefore, the research team's bioprinted 3D kidney architecture reproduces a very small – but very critical – subunit of the entire kidney.
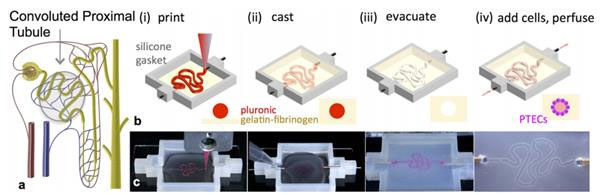
It is reported that Lewis's team used their earlier bio-printing living cells to form thick tissue to achieve this result. The following are the basic steps:
Using a customizable 3D printed silicone gasket as the mold, they initially "cast" a designed extracellular matrix as the base layer.
An "escaped ink" (which will eventually liquefy and be removed from the final structure) will then be printed into a complex, entangled tube shaped similar to the structure of the natural renal proximal tubule.
The printed structure is then encapsulated by another layer of extracellular matrix.
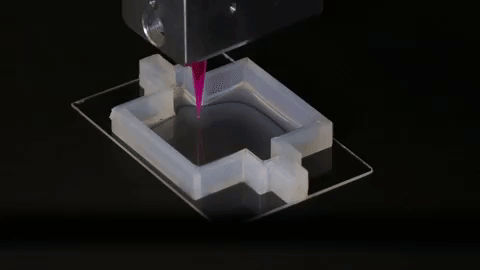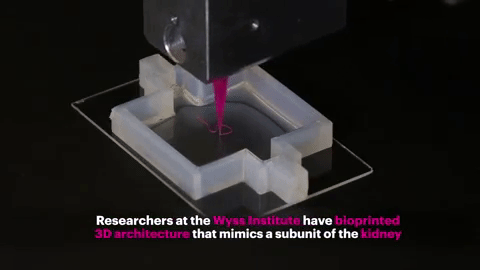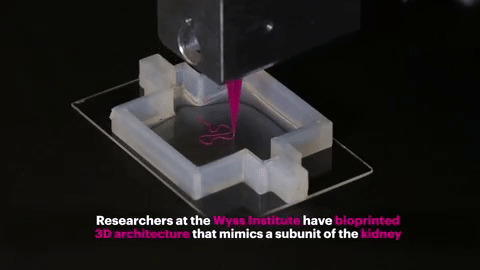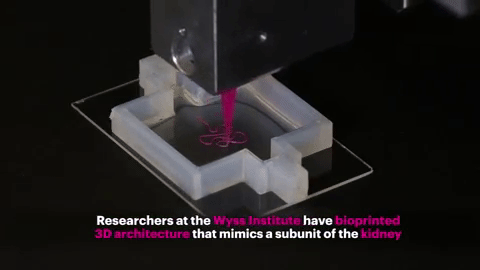
Finally, the entire structure is cooled and the "fugitive ink" is removed, resulting in an open tube embedded in the extracellular matrix.
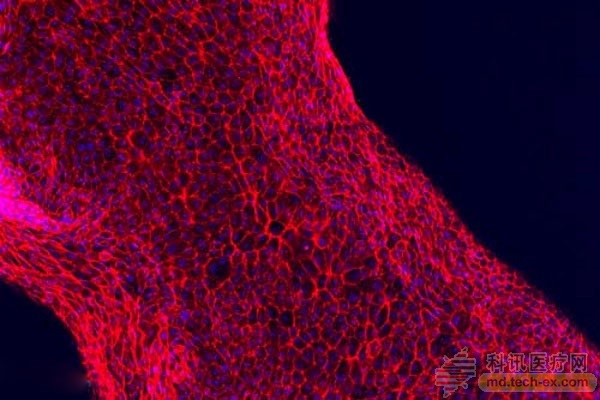
Next, the cell growth solution is perfused at the inlet and outlet of both ends of the tubule, and then the human proximal tubule cells are reperfused, and these cells quickly attach to the inner wall of the tube.
Eventually, these cells form a tightly packed monolayer that acts as a cellular barrier between the lumen of the tubule and the extracellular matrix outside.
During this process, nutrients are infused through the inlet and outlet of the tubules, nourishing the living cells and maintaining their activity and function for more than two months.
When the cells mature, the 3D kidney structure begins to perform the same important function as the natural renal proximal tubules. People can inject drugs or other substances into the 3D kidney structure to study their nephrotoxicity and overall effects on proximal tubule cells.
The co-first authors of the study, Kimberly Homan and David Kolesky, stressed that the most exciting aspect of this research is that it is a credible in vitro model and functions like a living kidney tissue with traditional 2D This is a huge improvement compared to cell culture.
Researchers say that in the long run, their approach can be further developed to make implants or organ aids. In the short term, it will also provide clinicians with a powerful tool for assessing treatment options or disease diagnosis based on patient specific characteristics, and is a very effective way to help pharmaceutical companies determine the impact of drugs on health and kidney function.
Moreover, as a manufacturing platform, this method is flexible, scalable, and adaptable. That is to say, using this method, the research team plans to explore other than to strive to create a larger kidney structure. Types of human tissues and organs.
(Compiled from Harvard University)
Source: Tiangongshe
Chitosan is a product of n-deacetylated chitosan. Chitin, chitosan and cellulose have similar chemical structure. Cellulose is hydroxyl group at C2 position, chitin and chitosan are replaced by an acetyl group and an amino group respectively at C2 position. Chitin and chitosan have many unique properties such as biodegradability, cell affinity and biological effect, especially chitosan containing free amino group, which is the only basic polysaccharide in natural polysaccharides.
The amino groups in the chitosan molecular structure are more reactive than the acetylamino groups in the chitin molecule, which makes the polysaccharide have excellent biological functions and can carry out chemical modification reaction. Therefore, chitosan is considered as a functional biomaterial with greater application potential than cellulose.
Chitosan is the product of natural polysaccharide chitin removing part of the acetyl group, with biodegradability, biocompatibility, non-toxicity, antibacterial, anti-cancer, lipid-lowering, immune enhancement and other physiological functions. Widely used in food additives, textile, agriculture, environmental protection, beauty care, cosmetics, antibacterial agent, medical fiber, medical dressings, artificial tissue materials, drug slow release materials, gene transduction carrier, biomedical fields, medical absorbable materials, tissue engineering carrier materials, medical and drug development, and many other fields and other daily-use chemical industry.
Chitin,Chitosan,Chitosan Oligosaccharide,Carboxymethyl chitosan
Allied Extracts Solutions , https://www.alliedadditives.com